Tested and fully functional. May require a new PDA. Cosmetic imperfections are present but do not affect performance. See photos for details of the cosmetic condition.
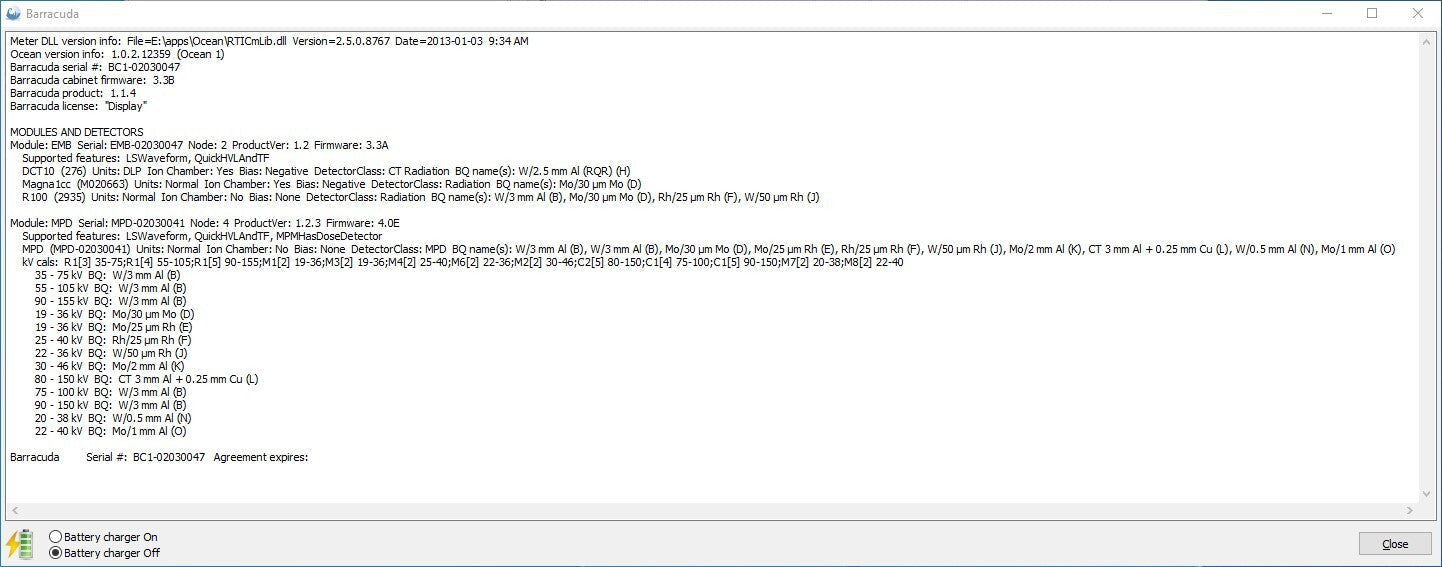
The Barracuda Cabinet, MPD and R100 have all been updated to the latest firmware versions via the RTI software.
A license is required to use the software, which can be acquired from RTI, although a 45 day trial period is available.
The PDA has also been updated and the QABrowser software installed onto it, however, probably due to its age, it is running into memory issues. We do not have the necessary experience to resolve this. Removing the batteries for more than a few seconds will result in all data being lost, requiring the device to be resynchronized.
The PDA is only required for operating the instrument without a PC, therefore the instrument can still be used with the included RTI Ocean software package.
All required software has been included on a USB flash drive for Windows. We performed the following steps to get everything up and running.
- Install the software in the following order (do not connect the instrument to the PC)
- Palm Desktop
- QA Browser
- CDM2136620 FTDI Drivers
- DVR_WINX64 (Only if FTDI Drivers are problematic)
- RTI2017.8C (Used only for updating the cabinet and accessories. This installs Ocean 2014, which cannot be used with the Barracuda)
- Ocean 2013 (This is the version that should be used with the Barracuda cabinet)
- Setup the PDA
- Connect the PDA to the PC via the HotSync Cable and USB to Serial converter.
- Open the Palm HotSync manager. If you get a message about it already running, then right click on it from the taskbar tray and click settings
- Go to Connections and enable serial
- Select the correct COM port from the dropdown menu (The correct port can be found in Windows Device Manager)
- You can increase the speed of synching by setting the Baud rate to 115200 in the Device Manager advanced settings.
- Once this is done, press the HotSync button on the cable and the device should begin to sync with the PC. This may take a while depending on the chosen baud rate.
- After the sync is complete, the PDA will prompt for a reset, after which the QABrowser software will be made available.
- Disconnect the PDA from the PC and mount it the Barracuda cradle. Tap the QABrowser app on the screen and wait for it to launch. The PDA is outdated and so the software is a bit flaky and may run into memory issues. As a side note, when you run the software initially, it may appear as though the PDA has crashed. Be patient and after a few minutes, the software will begin to run.
- Setup PC for RTI Ocean
- As mentioned before, Ocean 2014 is only required for applying the latest firmware to the instrument and its accessories. This has already been performed.
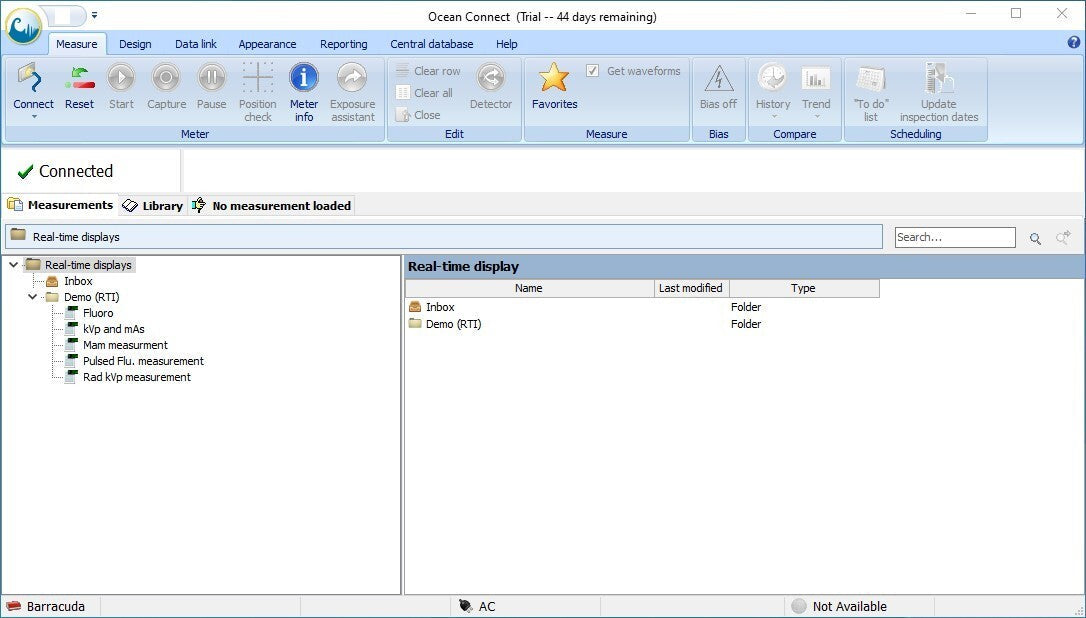
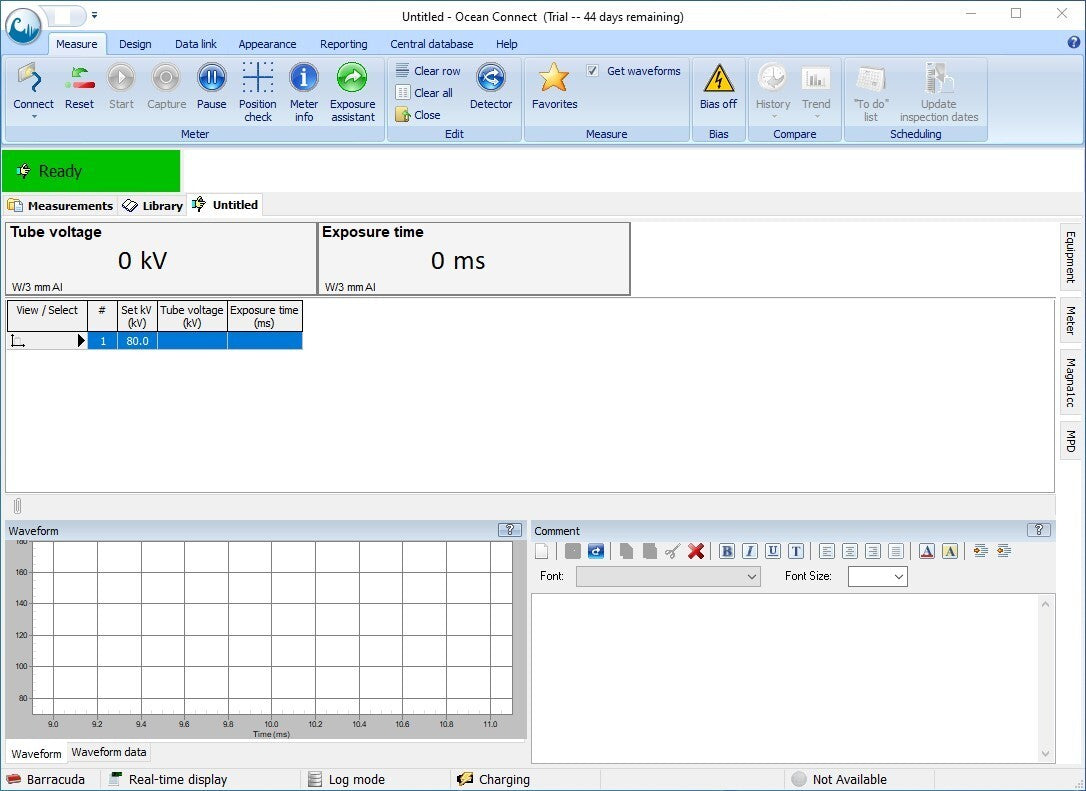
- To interact with the Barracuda, you will need to run the Ocean app.
- If you are using Windows 10 and above, you will need to run it in compatibility mode for Windows 7. In our experience, this is the only way to prevent it from randomly crashing.
- Interfacing with the Barracuda
- Once the Ocean software is up and running, plug the instrument into the PC via the included USB serial cable and connect to the device. Within a few seconds the status message should change from Connecting to Ready.
Included in the sale:
- RTI Barracuda Cabinet v1.1.4
- RTI Barracuda MPD Detector v1.2.3
- RTI Barracuda Dose Probe R100
- Palm TRG Pro 8022A PDA
- HotSync Cable
- FTDI USB to Serial converter
- Barracuda Cabinet power supply
- USB serial interface cable
- PDA cradle
- Long PDA cable
- Various attenuators
- Carry case
- USB flash drive with required software
This item may constitute medical equipment and is offered strictly in accordance with eBay UK and US policy requirements: by purchasing, you confirm that you are a licensed medical professional or otherwise authorised to acquire such equipment, that you will arrange for its inspection, calibration and certification by a qualified technician before any clinical or veterinary use, and that you will ensure full compliance with all applicable regulations—including, but not limited to, the UK Medicines and Healthcare products Regulatory Agency (MHRA) rules and US Food and Drug Administration (FDA) regulations under 21 CFR 801—prior to putting the device into service; you further acknowledge that the equipment is supplied “as-is”, with no warranty, guarantee of fitness for a particular purpose, or representation of current calibration status, and that the seller accepts no liability for any direct or consequential loss arising from improper use, resale, refurbishment, or failure to obtain the necessary approvals and certifications.